Commentary: Antituberculosis Drug Induced Fixed Drug Eruption- A Case Report
Jitendra H. Vaghela*
Senior resident, Department of Pharmacology, Government Medical College, Bhavnagar, Gujarat, India
Fixed Drug Eruption (FDE) is characterized by a single or multiple oval, erythematous patches due to systemic exposure to a drug that mostly resolves with a residual hyper pigmentation1. The overall incidence of FDE from FDC ranges from 3.77% to 15.34%2,3. Fixed drug eruption is one of the serious conditions affecting individual’s quality of life. Presently, it is known to occur due to many medications. Among the known medications, some antituberculosis drugs are also one of the causative drugs for fixed drug eruption4. Skin reactions due to some antituberculosis drugs are commonly found in patients with history of drug allergy5.
According to recent guidelines, use of fixed dose drug combinations (FDC) are promoted for its better compliance and advantages. World Health organization also advocates the use of FDCs. In India, under Revised National Tuberculosis Control Programme (RNTCP), from 1st April 2018 fixed dose drug combinations have been started for treatment of tuberculosis with daily given drug regimens. Previously thrice a week individual drug regimen was provided by the programme6. Fixed dose drug combinations have its own advantages. It decreases the number of tablets, frequency of tablets and also improves patient adherence to the treatment.
In present case, patient was prescribed FDC of antituberculosis drugs and developed FDE7. Fixed drug eruption (FDE) was caused by fixed dose combination (FDC) of antituberculosis drugs- tablet forecox (rifampicin 225 mg + isoniazid 150 mg + pyrazinamide 750 mg + ethambutol 400 mg) in 40-year-old male patient with previous history of drug allergy. Patient developed FDE after taking the third dose of tablet forecox for pulmonary tuberculosis. After taking the third dose, patient noted multiple, discrete, hyper pigmented patches on nape of neck; around mouth; both eyes; lower abdomen; back and upper abdomen. Patient was advised not to take antituberculosis drugs further and he was treated with injection dexamethasone 4 mg i.m. stat, then oral prednisolone 5 mg 6 hourly; injection ceftriaxone 1 gm i.v. 12 hourlies; framycetin cream for local application; mucaine viscous gel per orally 12 hourlies; betadine gargles and tablet multivitamins for 15 days. Tablet forecox was withdrawn and the reaction was recovered after 15 days of treatment for FDE. As per WHO-UMC and Naranjo’s causality assessment criteria, the association between reaction and tablet forecox was possible and probable, respectively8,9. The reaction was moderately (Level 4b) severe as per Modified Hartwig and Siegel’s scale10.
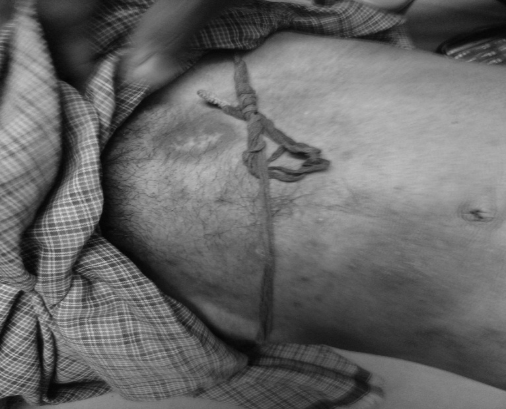
Figure 1: Fixed drug eruption lesion
Among patients from low socioeconomical class and having comorbid conditions, adverse drug reactions due to drugs may occur more frequently11. In present case, patient was from lower socioeconomical class, labourer, alcoholic and suffering from comorbidity. Patient has been prescribed antituberculosis drug therapy by private practitioner. Patient history revealed that he had some unknown drug allergy. But, it is one of the limitations of case report that no confirmation was possible for the drug to which patient was allergic. We were unable to perform the patch test for identifying the culprit drug. Patient was diagnosed to suffer from comorbid condition of HIV infection, so patient was started on antiretroviral therapy during the time of hospitalisation. This can be also considered as one of the limitations of the study for identifying the culprit drug.
To conclude, fixed drug dose combinations are widely accepted but before using such combinations for each and every patient one has to keep in mind the possibility of history of drug allergy.
References
- Paulmann M, Mockenhaupt M. Severe drug?induced skin reactions: clinical features, diagnosis, etiology, and therapy. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2015 Jul 1; 13(7): 625-43.
- Patel TK, Thakkar SH, Sharma DC. Cutaneous adverse drug reactions in Indian population: A systematic review. Indian dermatology online journal. 2014 Dec; 5(Suppl 2): S76.
- Siribaddana A, Dissanayake KS, Athukorala GP, et al. Frequency of Adverse Effects of Fixed Dose Combinations, in Tuberculosis and their Effects on Treatment Outcome. SAARC Journal of Tuberculosis, Lung Diseases and HIV/AIDS. 2009; 12(2): 8-12.
- Bakayoko AS, Kaloga M, Kamagate M, et al. Fixed drug eruption after taking ethambutol Rev Mal Respir. 2015; 32(1): 48-51.
- Arikoglu T, Aslan G, Batmaz SB, et al. Diagnostic evaluation and risk factors for drug allergies in children: from clinical history to skin and challenge tests. International journal of clinical pharmacy. 2015 Aug 1; 37(4): 583-91.
- Chaudhuri AD. Recent changes in technical and operational guidelines for tuberculosis control programme in India-2016: A paradigm shift in tuberculosis control. The Journal of Association of Chest Physicians. 2017 Jan 1; 5(1): 1.
- Vaghela JH, Nimbark V, Barvaliya M, et al. Antituberculosis Drug-Induced Fixed Drug Eruption: A Case Report. Drug safety-case reports. 2018 Dec 1; 5(1): 23.
- World Health Organization (WHO). Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. WHO, http://www. who-umc. org/graphics/4409. pdf. Google Scholar. 2017.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clinical Pharmacology & Therapeutics. 1981 Aug 1; 30(2): 239-45.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. American Journal of Health-System Pharmacy. 1992 Sep 1; 49(9): 2229-32.
- Odubanjo E, Bennett K, Feely J. Influence of socioeconomic status on the quality of prescribing in the elderly–a population-based study. British journal of clinical pharmacology. 2004 Nov 1; 58(5): 496-502.
