COVID-19 Disease as an Acute Angiotensin 1-7 Deficiency: A Preliminary Phase 2 Study with Angiotensin 1-7 in Association with Melatonin and Cannabidiol in Symptomatic COVID19 -Infected Subjects
Paolo Lissoni1*, Franco Rovelli1, Alejandra Monzon1, Giusy Messina1, Enrica Porta1, Giorgio Porro1, Sonia Pensato1, Elio Martin1, Andrea Sassola1, Alberto Caddeo1, Carla Galli1, Nicoletta Merli1, Agnese Valentini1, Giuseppe Di Fede1
1Institute of Biological Medicine, Milan, Italy
Abstract
COVID-19 disease is characterized by severe and acute immune alterations, consisting of an abnormal secretion of inflammatory cytokines, mainly IL-17, IL-6 and TNF-alpha, in association with decline in lymphocyte and increase in monocyte counts. ACE2 is the key for COVID19 entry into the cells. However, the link of viral spike protein to ACE2 receptor on cell surface would also block the ACE2 enzymatic activity itself, with a consequent diminished production of angiotensin 1-7 (Ang 1-7), which is provided by fundamental anti-inflammatory and anti-coagulant properties. Then, the severe and acute Ang 1-7 deficiency would allow an exaggerated cytokine-induced inflammatory response, endothelial damage, leak capillary syndrome and acute respiratory distress syndrome (ARDS). Moreover, because of the documented connections occurring among ACE2, cannabinoid system and melatonin (MLT) secretion from the pineal gland, the block of ACE2 activity would also allow a concomitant deficiency of pineal-cannabinoid system axis, which plays a fundamental anti-inflammatory role by inhibiting IL-17 secretion, one of the main cytokine involved in COVID19 infection. Therefore, COVID19-induced exaggerated inflammatory response could be controlled at least in part by correcting Ang 1-7, MLT and cannabinoid deficiency through their exogenous administration. On these bases, a study was planned in 30 COVID19-infected patients with initial or important symptomatology, 16 of whom orally treated by MLT (20 mg/day in the evening) plus cannabidiol (CBD) (10 mg x 2/day) only, while the other 14 patients received also Ang 1-7 (0.5 mg 2/day orally). The results were compared to those observed in a control group of 30 COVID-19 infected patient, who received the only supportive therapy. No hospitalisation for initial respiratory failure was required in the group of patients treated by the neuroimmune regimen. In addition, most patients referred a rapid disappearance of fever and myalgia, as well as a relief of asthenia, particularly in those concomitantly treated with Ang 1-7. On the contrary, 5/30 (17%) control patients required hospitalisation. The difference was statistically significant (0/30 vs 5/30, P< 0.05). This preliminary study would suggest that a neuroimmune approach consisting of MLT and CBD in association with Ang 1-7 is an effective and non-toxic regimen in the therapy of COVID19-related symptoms, which could also control the clinical evolution of disease and reduce the need of hospitalisation.
Background
It is known that ACE2 is the key for COVID-19 entry into the human cells. In addition, some preliminary results would also suggest that the link of viral spike protein to ACE2 on cell surface does not determine the only virus entry into the cells, but also a reduction of ACE2 on cell surface, even though by mechanisms which need to be better defined, involving endocytosis or shedding1,2. Since ACE2 is the enzyme responsible for Angiotensin 1-7 (Ang 1-7) generation, the decline in ACE2 expression and its biological activity would determine an acute and severe deficiency of Ang 1-7 itself1,2, with a consequent unbalance between angiotensin II (Ang II) and Ang 1-7, which are provided by opposite activities3-5. In fact, Ang 1-7 has been proven to exert hypotensive, anti-inflammatory, anti-proliferative, anti-thrombotic, anti-angiogenic, and anti-fibrotic activities. Moreover, Ang 1-7 could also prevent the development of an acute respiratory distress syndrome (ARDS)6,7, and thrombotic phenomena8, which represent the main complications of COVID19 infection. In contrast, Ang II has appeared to play hypertensive, inflammatory, proliferative, thrombotic, angiogenic and pro-fibrotic effects3-5. In addition, viral block of ACE2 activity would activate the fatty acid amide hydrolase (FAAH)9, the enzyme involved in the destruction of cannabinoids, with a following endocannabinoid system deficiency10. Then, because of the fundamental anti-inflammatory role of cannabinoid system11, endocannabinoid deficiency could furtherly amplify the COVID-19-induced exaggerated inflammatory response. The anti-inflammatory effects of cannabinoids would be mainly depend on their inhibitory effects on IL-17 secretion12, which would constitute the main inflammatory cytokine involved in COVID19 infection13., because of its stimulatory role on the secretion of macrophage-related inflammatory cytokines14, such as IL-6 and TNF-alpha. IL-17 has also appeared to exert direct cardiopulmonary toxic effects15, including ARDS16, endothelial damage with a consequent enhanced thrombotic risk17, and a stimulation of ACE expression in association with an inhibition of ACE2 expression18,19. Finally, because of the reciprocal stimulatory interactions occurring between endocannabinoid system and pineal gland20, the endocannabinoid deficiency could allow a concomitant pineal failure, with a consequent diminished production of its most known hormone melatonin (MLT), whose anti-inflammatory properties have been well documented21. Then, COVID19 disease may be considered as an acute and severe neuro-endocrine-immune pathology, since it involves both immune and neuroendocrine systems, with as end-result an acute Ang 1-7 deficiency. The physiopathology of COVID19 disease is synthetised in Figure 1. Therefore, according to this physio-pathological point of view, the main therapy of COVID19 disease could primarily consist of Ang 1-7 administration2,19. This statement is also justified by the fact that all conditions predisposing to the clinical complications of COVID19 disease, including hypertension, obesity, diabetes, age and male sex, are characterized by an already reduced endogenous production of Ang 1-71-5. In addition, further benefits could be achieved by also correcting MLT and cannabinoid deficiencies, since both MLT and cannabinoid agents, such as cannabidiol (CBD), have been proven to control the inflammatory response by inhibiting IL-17 secretion12,20 and FAAH activity12,21. In fact, it has been shown that a previous exposure to MLT may predict a better prognosis in intubated COVID19 infected patients22. On these bases, a preliminary study was planned with a neuroimmune regimen consisting of MLT and CBD, alone or in association with Ang 1-7 in a group of COVID19-infected symptomatic patients to evaluate its efficacy and tolerability.
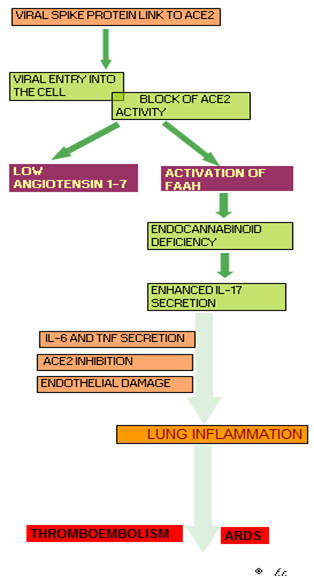
Figure 1.Pathogenesis of COVID-19 disease (FAAH: Fatty acid amide hydrolase; ARDS: Acute respiratory distress syndrome)
Patients and Methods
The study included 30 consecutive symptomatic COVID19-infected patients (M/F: 16/14; median age: 55 years, range 15-82), 16 of whom were treated only with MLT plus CBD, while the remaining 14 patients also received Ang 1-7. Eligibility criteria were, as follows: molecular diagnosis of COVID19 infection by RT-PCR assay, and presence of important symptoms, including fever higher than 37.5° C and myalgia. Important asthenia occurred in 17/30 (57%) patients. After the approval of the Ethical Committee, the experimental protocol was explained to each patient, and written consent was obtained. All drugs were orally administered. Ang 1-7 was given at 0.5 mg twice/day in gastro-protected capsules, MLT at 20 mg once day in the dark period, and CBD at 10 mg twice/day. According to the preliminary guidelines, the supportive care consisted only of paracetamol, expectorants, and low-dose dexamethasone. The treatment was rapidly begun on the onset of the first symptoms. All subjects presented fever greater than 37.5 C, asthenia, rhinorrhoea, mild or important myalgia, and reduced taste perception. The results were compared to those observed in an age- and sex-matched control group of 30 symptomatic COVID-19 infected subjects, who were followed during the same period, and who received the only supportive care,16/30 (53%) of whom referred an important asthenia. The immune status was evaluated by monitoring changes in lymphocyte-to-monocyte ratio (LMR), because of its well demonstrated prognostic significance to monitor the clinical evolution of COVID-19 disease23. Normal values of LMR observed in our laboratory (95% confidence limits) were greater than 2.1. Data were reported as mean +/- SE, and statistically analysed by the chi-square test and the Student’s t test, as appropriate.
Results
The characteristics of patients and their response to therapy are reported in Table 1. No subject treated with MLT plus CBD with or without a concomitant Ang 1-7 administration required hospitalisation for pulmonary or other important complications. Moreover, no therapy-related side effect occurred. On the contrary, fever and myalgia disappeared in all subjects within 3 days of treatment, while the disturbances of taste perception persisted for a longer period. Moreover, a rapid improvement of asthenia occurred in 8/17 (47%) patients, and the percentage of resolution of asthenia achieved in patients concomitantly treated with Ang 1-7 was significantly higher than in patients treated with MLT plus CBD alone (6/8(75%) vs 2/9(22%), P<0.05). In addition, from a psycho-emotional point of view, all subjects who received the neuroimmune regimen experienced a relief of anxiety, a better consciousness status, and an improvement in their sleep quality. On the other hand, 5/30 (17%) symptomatic subjects of the control group required hospitalisation for initial important respiratory symptoms to be treated by mechanic ventilation or intubation. Then, the percentage of hospitalisation observed in controls was significantly higher than that occurring in the treated group (5/30 vs 0/30, P<0.05). Moreover, the percentage of relief of asthenia achieved in the group of treated patients was significantly higher than that observed in the control group (8/17(47%) vs 2/16(13%), P<0.01). On the contrary, no significant difference was observed in the mean period required to achieve a negativity of COVID19 infection molecular diagnosis between treated patients and controls (15 +/ 3 days vs 18 +/- 4 days). Finally, from an immunological point of view, no decline in LMR ratio occurred on therapy in subjects treated by the neuroimmune regimen (2.9 +/- 0.3 vs 2.7 +/- 0.4). On the contrary, a rapid decline in LMR greater than 30%, due to both lymphocyte decrease and monocyte rise, occurred in all 5 controls subjects who required hospitalisation, whereas no decline was seen in the other 25 control subjects.
Table 1: Clinical characteristics of COVID-19-infected patients and their response to therapy
|
CHARACTERISTICS |
CONTROL GROUP |
MLT + CBD |
MLT + CBD + Ang 1-7 |
ALL TREATED PATIENTS |
|
|
( n=30) |
(n=16) |
( n=14) |
( n=30) |
|
M/F |
18/12 |
9/7 |
8/6 |
17/13 |
|
MEDIAN AGE (years) |
58 (16-76) |
54 (16-82) |
56 (15-78) |
55 (15-82) |
|
PRESENCE OF ASTHENIA |
16/30 (53%) |
9/16 (56%) |
8/14 (57%) |
17/30 (57%) |
|
HOSPITALISATION |
5/30 (17%) |
0/16 |
0/14 |
0/30* |
|
RELIEF OF ASTHENIA |
2/16 (13%) |
2/9 (22%) |
6/8 (75%)*** |
8/17 (47%)** |
*P<0.05 vs controls; ** P< 0.01 vs controls; ***P< 0.05 VS MLT + CBD alone+ MLT: melatonin; CBD: cannabidiol; Ang 1-7: angiotensin 1-7.
Discussion
This preliminary study would suggest that a neuroimmune approach carried out to control host inflammatory response by using the most known active neuroendocrine anti-inflammatory molecules, consisting of the pineal hormone MLT, CBD and in particular Ang 1-7, whose deficiency would be one of the mechanisms responsible for COVID 19 pathology, may counteract the clinical complications of COVID-19 infection. This finding is not surprising, since the previous exposure to MLT has been proven to prevent COVID 19-induced respiratory complications22. In addition, CBD has appeared to inhibit IL-17 secretion12, and Ang 1-7 has been shown to opposite both lung failure and thromboembolic phenomena3-8. Then, this evidence would further confirm the role played by the deficiency of Ang 1-7 production and the decreased anti-inflammatory activity of the pineal-cannabinoid system functional axis to counteract the clinical evolution of COVID-19 disease. Moreover, this preliminary results would show that the occurrence of a rapid decline in LMR values, due to both lymphocyte count decline and monocyte number increase, may predict the evolution into respiratory failure depending on an exaggerated inflammatory response. Obviously, the too number of subjects considered in the study does not allow us to draw defined conclusions. However, in any case these preliminary results would justify further randomised clinical studies in COVID19-infected subjects treated with the common palliative therapy alone or in association with a concomitant neuroimmune approach. Moreover, further studies by monitoring changes in the blood levels of the main inflammatory cytokines, including IL-17A, IL-6 and TNF-alpha, as well as those of MLT and Ang 1-7 itself, will be required to better define the mechanisms involved in the possible control of COVID19-related inflammatory response. In any case, in this moment the main planetary problem is to reduce the need of hospitalisation for COVID19 infection. The neuroimmune regimen with Ang 1-7, MLT and CBD could contribute to achieve a diminished request of hospitalisation for COVID 19 infection in positive symptomatic patients. In conclusion, in addition to the fundamental role played by the prevention of the infection by the interpersonal distance and mask, this study suggests that another way to control the pandemic diffusion of COVID 19 is to act on the biological response of subjects by enhancing their anti-inflammatory potency.
References
- Lanza K, Perez LG, Costa LB, et al. Covid-19: the renin-angiotensin system imbalance hypothesis. Clin Sci (Lond). 2020; 134: 1259-1264.
- Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Int Med. 2020; 76: 14-20.
- Capettini LS, Montecucco F, Mach F, et al. Role of renin-angiotensin system in inflammation, immunity and aging. CurrPharmDes. 2012; 18: 963-970.
- Rodriguez-Prestes TR, Pessoa Rocha N, Silva Miranda A, et al. The anti-inflammatory potential of ACE2/Angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets. 2017; 18: 1301-1313.
- Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/Angiotensin-(1-7)/Mas axis of the renin-angiotensin system: focus on Angiotensin-(1-7). Physiol Res. 2018; 98: 505-553.
- Patel VB, Takawale A, Ramprasath T, et al. Antagonism of angiotensin 1-7 prevents the therapeutic effects of recombinant human ACE2. J Mol Med (Berlin). 2015; 93: 1003-1013.
- Peirò C, Moncada S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Ciirculation. 2020; 141: 1665-1666.
- Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, et al. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effects. Clinics. 2011; 66: 837-841.
- Lissoni P, Rovelli F, Pelizzoni F, et al. Coronavirus-induced severe acute respiratory syndrome (SARS) as a possible expression of fatty acid amide hydrolase (FAAH) hyper-function and possible therapeutic role of FAAH inhibitors in Covid 19-induced SARS. J Clin Res Rep 5: DOI:10.31579/2690-1919/108.
- Russo EB. Clinical endocannabinoid deficiency (CECD). Neuroendocrinol Lett. 2020; 25: 31-39, 2004.
- Grotenhermen F. Pharmacology of cannabinoids. Neuroendocrinol Lett. 2004; 25: 14-23.
- Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009; 1: 1333-1349.
- Casillo GM, Mansour AA, Raucci F, et al. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol Res. 2020; 156: 104791. doi: 10.1016/j.phrs.2020.104791.
- Kryczek I, Wei S, Vatan L, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007; 179: 1423-1426.
- Megna M, Napolitano M, Fabbrocini G. May IL-17 have a role in COVID-19 infection? Med Hypotheses. 2020; 140: 109749.
- Li Q, Gu Y, Tu Q, et al. Blockadeof interleukin-17 restrains the development of acute lung injury. Scand J Immunol. 2015; 83. htt://doi.org/10.1111/sji.12408.
- Robert M, Miossec P. Effects of interleukin-17 on the cardiovascular system. Autoimmun Rev. 2017; 16: 984-991.
- Madhur MS, Lob HE, McCann LA, et al. Interleukin17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010; 55: 500-507.
- Shete A. Urgent need for evaluating agonists of angiotensin-(1-7)/Mas receptor axis for treating patients with COVID-19. Int J Infect Dis. 2020; 96: 348-351.
- Lissoni P, Rovelli F, Messina G, et al. A review on the neuroendocrine regulation of cytokine secretion: possible modulation of the cytokine network by the pineal hormone melatonin and cannabidiol. Oncol Res Rev. 2019; 2: 1-4.
- Kuklina EM, Glebezdina NS, NekrasovaIV. Role of melatonin in the regulation of differentiation of T cells producing interleukin-17 (Th17). Bull Exp Biol Med. 2016; 160: 656-658.
- Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. Version 1. medRxiv. Preprint. doi: 10.1101/2020. 10.15.20213546.
- Lissoni P, Rovelli F, Monzon A, et al. Evidence of abnormally low lymphocyte-to-monocyte ratio in Covid-19-induced severe acute respiratory syndrome. J ImmunoAllerg. 2020; 1: 1-6.
