Electro-Filtering Paper Spray Ionization Method
Fangling Wu1, 2, Chuan-Fan Ding1, 2*
1Institute of Mass Spectrometry, School of Materials Science & Chemical Engineering, Ningbo University, Ningbo, Zhejiang, China
2Department of Chemistry, Fudan University, Shanghai, China
Abstract
A relatively rigid and good electrical conductive copper filter severed as a substrate for the paper spray named Electro-Filtering Paper Spray Ionization (EFSI) is mini reviewed. The copper filter is used to increase the conductivity and pressure tolerance for the paper substrate, which can insure sufficient, efficient and direct sample-solvent extraction for different types of solid samples, and also a low voltage is required for paper spray. The capability and reliability of EFSI-MS were demonstrated experimentally for indirect high-throughput component analysis or the desired ion signals for a wide range of solid samples by selecting and optimizing different extraction solvents, which is not feasible with conventional ESI or direct paper spray methods. Simultaneously, trace targeted substance for the large-volume sample can be effective extracted and pre-concentration by the optimized solvent for direct mass spectrometry analysis with no or extremely minimal sample preparation effort for the EFSI method. Besides, with the attractive features of cost-effectiveness, rapid, process simplicity and high-throughput, the potential and novel applications of the EFSI-MS or EFSI-MS-MS can focused in the fields of environment, bio-science, food safety, and in-vitro diagnostics in clinical routine analysis are expected in the future work, where substantial time and labor could be saved when investigating the potential causes of disease.
Introduction
Mass spectrometry (MS) is a powerful analysis technology, providing information on molecular weight and chemical structures of the analytes in different field1-3. Ion source is a critical part of the MS, and for the primal and widely used ion source such as electrospray ionization (ESI)4, 5, complex sample preparation or routinely chromatography separation is required for the complex practical samples before the MS measurement. However, the development of desorption electrospray ionization (DESI) by Cooks et al. in 20046, and then the introduction of direct analysis to real time (DART) by Cody and co-workers in 20057, which were the pivotal milestones in the development of a subfield of MS analytical known as ambient ionization MS (AI-MS)8, 9. This new ionization method of AI-MS has greatly altered the traditional analysis that allowing sample analysis requiring no or minimal sample preparation, and it takes place in the atmospheric pressure in a quick, simple, and effective manner. Then, many other sub-group of AI methods were developed to address the need for rapid and direct analysis of practical samples subsequently, such as desorption atmospheric pressure chemical ionization (DAPCI)10, plasma-assisted desorption/ionization (PADI)11, dielectric barrier discharge ionization (DBDI)12, atmospheric pressure solids analysis probe (ASAP)13, electrospray-assisted laser desorption ionization (ELDI)14, matrix-assisted laser desorption electrospray ionization (MALDESI)15, laser ablation-electrospray ionization (LAESI)16, and infrared laser-assisted desorption electrospray ionization (IR-LADESI)17. In addition, many other new AI methods characterized by the generation of an electrospray directly from the sample were investigated, such as paper spray electrospray ionization (PS)18-21, extractive electrospray ionization (EESI)22, wooden-tip electrospray ionization23, and ultrasonic surgical aspiration and sonic spray24.
The aforementioned AI methods, PS is interesting described as a direct ionization method employing cellulose based materials of paper, first developed by Cooks and Ouyang groups in 201019. PS is built on a piece of triangular paper, held by a metal clip and positioned in front of the MS orifice. Analytical sample is placed on the middle of the paper triangle, and the electrospray ionization generated by wetting the paper substrate with solvent. Overall, the PS-MS method shows great promises for quantitative analyses for a number of applications, due to the high matrix tolerance and simple operation, especially for the illicit drugs in blood sample 20. However, some weaknesses have existed on the method of PS, such as it was easily deformed when subjected to high pressure that it cannot bear a large volume sample. Paper electrospray is the ionization of the analytes by conduction through a solvent on the paper substance, which the paper should be wetted during the analysis process. In this way, some other AI methods were developed based on PS method, such as aluminum foil electrospray ionization25, leaf spray26, and paper cone spray ionization27.
Electro-Filtering Paper Spray Ionization
Electro-Filtering Paper Spray Ionization (EFSI) method was also developed on based of PS and validated by our group28, 29, which a metal filter is served as substrate for the paper spray. The scheme of the EFSI described in Figure 1c29. A trungkoni-like filter shape was papered by copper sheet, with 12 mm for height, 10 mm for top radius and 5 mm for radius at the base. A piece of chromatography paper cut into a triangular shape with 30° angles was held by the copper filter, and the length of the paper to the copper top filter was 5 mm. The tip of the paper was directly facing the inlet of the mass spectrometer, the EFSI device was oriented approximately 30° to the horizon, and the distance between the filter-paper tip and the MS inlet was 3-5 mm. Sheath gas (nitrogen, 99.999%) at a flow rate of 0.2–0.5 L min−1 was used to facilitate the solid-liquid extraction process and allow rapid infiltration of the analytes into the chromatography paper for separation and spray analysis.
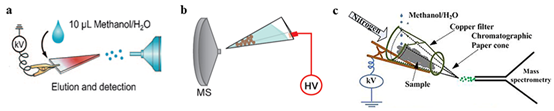
Figure 1: Structural of the (a) paper spray21; [b] paper cone spray ionization27; [c] Copper Electro-filtering spray ionization source29
Some distinct advantages can be described for the method of EFSI, such as economic, quick, and high-throughput analysis for large-volume unprocessed practical mixtures. Meanwhile, good electrical conductivity of the electro-filtering made by metal sheet severs as substrate for the paper, resulting in a low voltage was required for paper spray and no solvent was required to wet the paper substance compared to separated paper spray method (Figure 1a)21 and paper cone spray ionization (Figure 1b)27. Analytical sample was incubated in the electro-filter, where a big conductive area for the analysis sample, and the paper spray can be strengthened. Besides, enough interspace for sample incubation can avoid the clogging problems for the EFSI method, which was often encountered in capillary based ESI. As it well known that, trace substance analysis often needs high sensitivity instrument or the complex sample pre-concentration process before detection30. Additionally, relatively rigid copper filter can sustain large size practical sample for direct sample-solvent extraction, the trace substance can be released and pre-concentration from the sample for MS analyzed directly.
Influencing factors on the performance of the Electro-Filtering Paper Spray Ionization method
For EFSI, a sheet metal was prepared as an Electro-Filtering and served as substrate for paper spray to bear more pressure for analytical sample, as well as strengthen the conductivity of the paper spraying. Moreover, sample-solution extraction processing can be realized for loading samples of the EFSI owing to the metal electro-filter. Variation in absolute signals complied with using different types of iron sheet, aluminum sheet, and copper sheet, in which EFSI prepared by copper sheet yielded the best MS signal intensity. This is mainly because copper sheet possesses the highest electrical conductivity and it was hard to be oxidized compared to iron sheet and aluminum sheet31, resulting from the EFSI prepared by copper sheet corresponds to higher ionization efficiency. Analogous to the PS method32, it was demonstrated that the paper types also influenced to the spray or detection signal of the analytes by the EFSI method. Various paper substances of filter paper with different pore sizes, weight paper and chromatography paper, in which chromatography paper was regarding the optimum choice for the most favorable physical and chemical properties for obtaining the lowest chemical noise and best MS spectra. Besides, types of extraction solvent, solvent volume and extraction frequency are also impacting the detection results. Extraction solvent is not only effect on the release of analytes from complex sample matrices, but also important to spray efficiency for the analytes. In general, organic solvent mixed with water is commended to be used as an extraction solvent33, in which the commonly used organic solvents are methanol, acetonitrile, acetone, dichloromethane etc.34,35. According to the theory, the extraction solvent should be as small as possible to eliminate reductions in analyte distribution constants, and the organic solvent content should above or equal to 50 vol%, which is beneficial for the sample spray and to ensure the high detection sensitivity. Extraction frequency is one of the most crucial steps related to extraction time, and extraction frequency is always a compromise between the solvent volume, sensitivity and reproducibility of the analytical method. Some other parameters, such as sheath gas (N2) flow rate and the electro-filtering temperature can also affect the extraction efficiency. Typically, it is necessary to optimize the favorable extraction condition by experiment properly according to the diverse analytical sample.
Applications of the Electro-Filtering Paper Spray Ionization-MS
EFSI-MS shows great promise for quantitative and qualitative analyses for different practical samples, including target and non-target compounds of small biomolecules, protein or polypeptide molecules in solid, liquid, and powder samples. At present, EFSI-MS was success to analyze target analytes, such as antibiotic in complex animal products, reserpine and Cytochrome C in soil sample28,29. Evaluating the results from the EFSI-MS method were better consistency with the ESI result, and the precision obtained for reproducibility was 81.6%–96.3%, and recoveries were 75.0%–94.6%. Simultaneously, EFSI-MS was also applied to the non-target analysis in practical samples of apple, pork, coffee, and green tea (dried), in which good MS spectra of vitamins, tannins, mineral salts, organic acids, and carbohydrates (molecular weight within 400) were detected for apple; some multivalent compounds of polypeptide or protein in the pork was detected in pork; the main adduct ions of coffee ingredients such as caffeine and dipotassium phosphate or arginine were detected in coffee sample; by changing the extraction solvent, different MS spectra were appeared for the green tea (dried) of method/water and acetone/water. The result reveals that the mass of the target compounds in practical samples can be obtained by optimizing the extraction solvent. In addition, the method of EFSI-MS can avoid the possibility of loss or contamination of analytes during sample pre-treatment, and the method also displays accept accuracy and stability. Because EFSI can withstand large volume samples, that it can be applied to trace substance analysis by adding the analytical sample volume. Besides, low ionization voltage and simple device are required for the EFSI method, that it can be applied to vivo analysis by combined with mini mass spectrometer36. Otherwise, the potential and novel applications of the EFSI-MS or EFSI-MS-MS can focus in the fields of proteomics, metabolomics, biomarker discovery, and in-vitro diagnostics in clinical routine analysis are expected in the future work, where substantial time and labor could be saved when investigating the potential causes of disease.
Conclusion
In this mini review, the incorporate of a relatively rigid and good electrical conductivity copper filter for loading different types of analytical samples for effective sample-solvent extraction processing, as well as to strengthen the conductivity of the paper spraying. The method is applicable for indirect analysis of large-volume unprocessed samples, which cannot be achieved through conventional ESI or direct paper spray methods. Besides, EFSI-MS can generate the desired ion signals for a wide range of compounds by selecting and optimizing an appropriate extraction solvent. And the trace substance direct analysis for large volume sample can be realized by efficient and alternative sample-liquid extraction process. Additionally, with the attractive features of rapid analysis, high-throughput, cost-effectiveness, and process simplicity, that the method of EFSI-MS can be a potential alternative for effective targeted or component trace analysis of unprocessed practical samples by adding the sample volume, or even the biological tissue samples in clinical routine analysis.
References
- Bantscheff M, Schirle M, Sweetman G, et al. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem. 2007; 389: 1017-1031.
- Harris GA, Galhena AS, Fernandez FM. Ambient sampling/ionization mass spectrometry: applications and current trends. Anal Chem. 2011; 83: 4508-4538.
- Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006; 312(5771): 212-217.
- Fenn JB, Mann M, Meng CK, et al. Electrospray ionization–principles and practice. Mass Spectrom Rev. 1990; 9(1): 37-70.
- King R, Bonfiglio R, Fernandez-Metzler C, et al. Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectr. 2000; 11(11): 942-950.
- Takats Z, Wiseman JM, Gologan B, et al. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004; 306(5695): 471-473.
- Cody RB, Laramée JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005; 77(8): 2297-2302.
- Cooks RG, Ouyang Z, Takats Z, et al. Ambient mass spectrometry. Science. 2006; 311(5767): 1566-1570.
- Huang MZ, Yuan CH, Cheng S, et al. Ambient ionization mass spectrometry. Annu Rev Anal Chem. 2010; 3: 43-65.
- Chen H, Zheng J, Zhang X, et al. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J Mass Spectrom. 2007; 42(8): 1045-1056.
- Ratcliffe LV, Rutten FJ, Barrett DA, et al. Surface analysis under ambient conditions using plasma-assisted desorption/ionization mass spectrometry. Anal Chem. 2007; 79(16): 6094-6101.
- Na N, Zhao M, Zhang S, et al. Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J Am Soc Mass Spectr. 2007; 18(10): 1859-1862.
- Bruns EA, Perraud V, Greaves J, et al. Atmospheric solids analysis probe mass spectrometry: a new approach for airborne particle analysis. Anal Chem. 2010; 82(14): 5922-5927.
- Shiea J, Huang MZ, HSu HJ, et al. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun Mass Sp. 2005; 19(24): 3701-3704.
- Karas M, Bahr U, Gießmann U. Matrix-assisted laser desorption ionization mass spectrometry. Mass Spectrom Rev. 1991; 10(5): 335-357.
- Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem. 2007; 79(21): 8098-8106.
- Rezenom YH, Dong J, Murray K. Infrared laser-assisted desorption electrospray ionization mass spectrometry. Analyst. 2008; 133(2): 226-232.
- Espy RD, Muliadi AR, Ouyang Z, et al. Spray mechanism in paper spray ionization. International Journal of Mass Spectrometry. 2012; 325: 167-171.
- Liu J, Wang H, Manicke NE, et al. Development, characterization, and application of paper spray ionization. Anal Chem. 2010; 82(6): 2463-2471.
- Manicke NE, Yang Q, Wang H, et al. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int J Mass Spectrometry. 2011; 300(2-3): 123-129.
- Wang H, Liu J, Cooks RG, et al. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew Chem Int Edit. 2010; 49(5): 877-880.
- Chen H, Venter A, Cooks RG. Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation. Chem Commun. 2006; (19): 2042-2044.
- Hu B, So PK, Chen H, et al. Electrospray ionization using wooden tips. Anal Chem. 2011; 83(21): 8201-8207.
- Schäfer KC, Balog J, Szaniszló T, et al. Real time analysis of brain tissue by direct combination of ultrasonic surgical aspiration and sonic spray mass spectrometry. Anal Chem. 2011; 83(20): 7729-7735.
- Hu B, So PK, Yao ZP. Electrospray ionization with aluminum foil: a versatile mass spectrometric technique. Anal Chim Acta. 2014; 817: 1-8.
- Liu J, Wang H, Cooks RG, et al. Leaf spray: direct chemical analysis of plant material and living plants by mass spectrometry. Anal Chem. 2011; 83(20): 7608-7613.
- Kim, P, Cha S. Paper cone spray ionization mass spectrometry (PCSI MS) for simple and rapid analysis of raw solid samples. Analyst. 2015; 140(17): 5868-5872.
- Chen Y, Xu C, Ding CF, et al. Electro-filtering spray ionization source for soil analysis. Anal Lett. 2016; 49(2): 282-289.
- Wu FL, Chu YQ, Ding CF, et al. Antibiotic analysis using Electro-Filtering Paper Spray Ionization. Talanta. 2018; 190: 110-118.
- Lacina O, Hradkova P, Pulkrabova J, et al. Simple, high throughput ultra-high performance liquid chromatography/tandem mass spectrometry trace analysis of perfluorinated alkylated substances in food of animal origin: milk and fish. J Chromatogr A. 2011; 1218(28): 4312-4321.
- Lu L, Shen Y, Chen X, et al. Ultrahigh strength and high electrical conductivity in copper. Science. 2004; 304(5669): 422-426.
- Lai PH, Chen PC, Liao YW, et al. Comparison of gampi paper and nanofibers to chromatography paper used in paper spray-mass spectrometry. Int J Mass Spectrom. 2015; 375: 14-17.
- Russell WK, Park ZY, Russell DH. Proteolysis in mixed organic−aqueous solvent systems: applications for peptide mass mapping using mass spectrometry. Anal Chem. 2011; 73(11): 2682-2685.
- Liu YS, Ying GG, Shareef A, et al. Simultaneous determination of benzotriazoles and ultraviolet filters in ground water, effluent and biosolid samples using gas chromatography–tandem mass spectrometry. J Chromatogr A. 2011; 1218(31): 5328-5335.
- Gineys N, Giroud B, Vulliet E. Analytical method for the determination of trace levels of steroid hormones and corticosteroids in soil, based on PLE/SPE/LC-MS/MS. Anal Bioanal Chem. 2011; 397(6): 2295-2302.
- Li L, Chen TC, Ren Y, et al. Mini 12, Miniature mass spectrometer for clinical and other application introduction and characterization. Anal Chem. 2014; 86(6): 2909-2916.
