Linezolid-Associated Black Tongue: A Rare Adverse Drug Reaction
Dr. Gyanshankar P. Mishra
Associate Professor, Dept. of Respiratory Medicine, Indira Gandhi Government Medical College, C.A. Road, Nagpur, Maharashtra, India– 440018;
Abstract
Linezolid is an effective second-line anti-tuberculosis drug. However, the use of linezolid has been associated with rare adverse drug reactions (ADRs), one of which is the linezolid-associated black hairy tongue (BHT). This reaction is characterized by a black or dark brown discoloration of the tongue, which may be accompanied by a metallic or bitter taste. In this case report, we present a 19-year-old male patient who developed linezolid-associated black tongue while receiving linezolid as part of an all-oral, longer-course treatment regime for multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB). The patient's BHT was only cosmetic and resolved upon discontinuation of linezolid. This case report aims to raise awareness of this rare ADR and the importance of close monitoring and potential withdrawal of linezolid to optimize treatment outcomes.
Introduction
Tuberculosis (TB) is a major global health problem, and the emergence of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) has made the treatment of TB increasingly challenging1. Conventional treatment regimens for MDR-TB are associated with significant drawbacks, including high costs, long treatment duration, and significant side effects. In recent years, newer drugs such as bedaquiline and linezolid have been ranked among the most efficacious second-line anti-TB drugs (Group A drugs) and have been used as part of all-oral, longer-course treatment regimes for MDR/RR-TB2. Linezolid, an oxazolidinone class antibiotic, targets the bacterial ribosome and prevents the formation of a functional initiation complex, which is necessary for synthesizing bacterial proteins. Despite its effectiveness as a second-line drug for treating TB, linezolid has been associated with several adverse drug reactions (ADRs). One of the rarest ADRs of linezolid is the development of a black hairy tongue (BHT), also known as lingua villosa nigra3. This case report presents a 19-year-old male patient who developed BHT while receiving linezolid as part of an all-oral, longer-course treatment regime for multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB). This case report aims to increase awareness of linezolid-associated BHT as a rare ADR and emphasize the importance of close monitoring and potential withdrawal of linezolid to optimize treatment outcomes. This case report adds to the existing literature on linezolid-associated BHT. It serves as a reminder of the importance of monitoring ADRs in patients receiving linezolid for the treatment of TB.
Case report:
A 19-year-old male patient weighing 42.3 kg was diagnosed with rifampicin-resistant tuberculosis of the spine and initiated on a bedaquiline-based all-oral, longer-course treatment regime for MDR/RR-TB on November 4, 2022. The rifampicin resistance diagnosis was based on the CBNAAT (Cartridge-based Nucleic Acid Amplification) test for TB on the pus drained from the spinal abscess. The treatment regime comprises 18 to 20 months of levofloxacin, linezolid, clofazimine, & cycloserine. Bedaquiline is administered for the first six months. The dose of linezolid is 600 mg once a day for patients weighing more than 30 kgs and 300 mg once daily for persons weighing 16-29 kgs. Linezolid is tapered from 600 mg to 300 mg once a day after the initial six months of treatment. Pyridoxine 100 mg once daily is administered to all patients weighing more than 30 kgs and 50 mg once daily to all patients in weight-band of 16-29 kgs.
The patient received linezolid 600 mg orally once daily as part of the treatment regimen. After one week of treatment, the patient began to develop blackish discoloration of the tongue. The discoloration was only cosmetic and did not cause pain or discomfort when eating. The patient's taste sensation was unaltered. The discoloration would clear with brushing but would reappear again. It persisted at one month of treatment (Figure 1). Linezolid was continued with close monitoring as it was an essential core drug in the regime contributing to the regime's efficacy and as the patient did not experience any functional discomfort with the ADR being only cosmetic in the patient. After one and a half months, based on the final culture drug sensitivity report, the anti-TB treatment regime was changed, and linezolid was withdrawn, following which the blackish discolouration of the tongue resolved in two weeks (Figure 2). The causality of this linezolid-associated ADR assessed by Naranjo causality score was "probable ADR," with a score of 7.
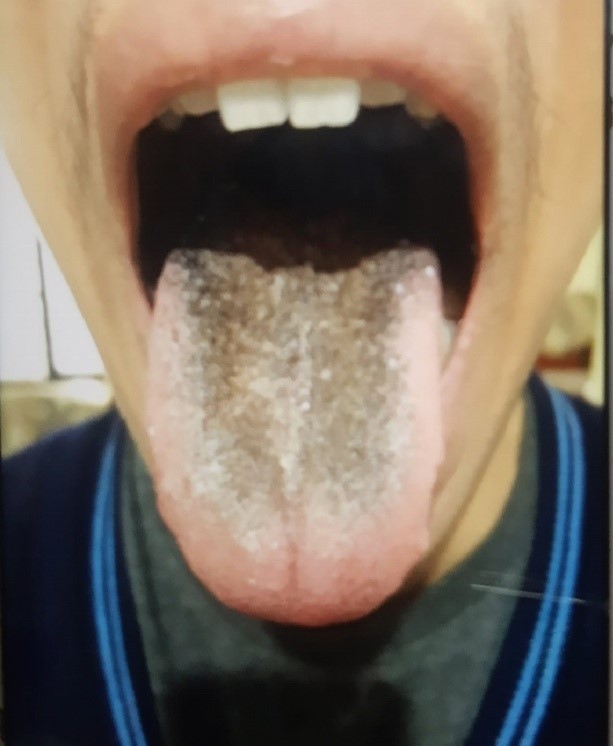
Figure 1: Linezolid-associated black hairy tongue after one month of treatment with linezolid.
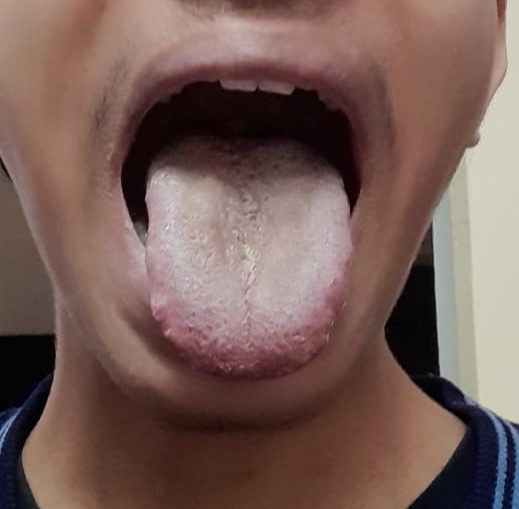
Figure 2: Symptomatioc resolution of the linezolid associated black hairy tongue after two weeks of stopping linezolid.
Discussion:
Dr. Amatus Lusitanus first described the phenomenon of BHT in 1557. BHT is a benign condition characterized by hypertrophied filiform lingual papillae with black discoloration of the dorsum of the tongue. It is caused by various drugs, including antibiotics (such as penicillins, tetracyclines, macrolides, linezolid, and metronidazole), antipsychotics, antidepressants, antineoplastic agents, and other drugs such as methyldopa3. Although BHT is usually asymptomatic, some patients may experience halitosis, nausea, tickling, and dysgeusia. The condition is caused by defective desquamation, and factors such as male sex, old age, smoking, alcohol use, and poor oral hygiene are considered predisposing factors. Medications such as cephalosporins, penicillins, sulfonamides, methyldopa, and linezolid have been reported to cause black discoloration of the tongue3. However, it is important to note that the BHT is mostly a benign and self-limiting reaction that resolves upon discontinuing linezolid. Thus, it is crucial to closely monitor patients for this and other ADRs and consider the potential withdrawal of linezolid if necessary to optimize treatment outcomes. Linezolid-associated BHT is a rare adverse drug reaction reported in patients receiving linezolid for treating tuberculosis4. Linezolid is an antibiotic that belongs to the oxazolidinone class of drugs and works by binding to the ribosome and preventing the formation of a functional initiation complex, which is necessary for synthesizing bacterial proteins. This inhibition of protein synthesis may lead to changes in the composition and structure of the oral mucosa, including the tongue, which can result in the development of BHT. It has been proposed that defective desquamation of keratinized layer of the tongue leads to excessive growth and thickening of filiform papillae of the tongue, which leads to a collection of microorganisms or foreign material5. However, the exact mechanism behind drug-induced BHT is not known.
BHT is a spontaneously resolving benign condition. The management of BHT is primarily supportive, with an emphasis on maintaining good oral and dental hygiene. In cases where the condition persists, gentle cleansing with a soft toothbrush and baking soda or hydrogen peroxide may be beneficial. Other topical agents include trichloroacetic acid, urea solution, thymol, gentian violet, and Vitamin B3.
In some cases, fungal cultures have been reported to be positive, leading to the use of antifungals. However, such interventions are typically not indicated, and the offending drug should be discontinued if possible. In instances where the drug is deemed essential for treating the underlying condition, reintroducing the drug may be considered after proper consultation with the treating physician3.
It is important to closely monitor patients for this and other ADRs and consider withdrawal of the offending drug if necessary to optimize treatment outcomes.
In the case presented here, the BHT was only cosmetic and did not cause any discomfort or alteration in taste sensation. As such, it was decided to continue linezolid treatment, considering the cosmetic nature of ADR without any functional affection. However, on withdrawal of linezolid due to a change of regime guided by the culture drug sensitivity report, the ADR subsided. Thus, it is important to closely monitor patients for this and other ADRs and consider the potential withdrawal of linezolid on account of ADR if necessary due to any functional impairment. This is important to optimise treatment outcomes.
Linezolid, a member of the oxazolidinone class of antibiotics, has been demonstrated to combat gram-positive bacterial infections effectively. Additionally, it has been widely adopted as a second-line drug in treating tuberculosis (TB)6. As a component of current anti-TB treatment regimens, linezolid has been utilized to shorten the duration of treatment for patients with drug-resistant TB7. Despite its therapeutic efficacy, linezolid usage has been linked to a spectrum of ADRs, including peripheral neuropathy, lactic acidosis, anemia, optic neuritis, and the rare condition of black BHT3,6.
In conclusion, this case report highlights the rare but clinically significant ADR of BHT associated with linezolid therapy for treating multidrug-resistant tuberculosis. The exact mechanism of this reaction remains unclear but is thought to be related to the inhibition of protein synthesis by linezolid. It is crucial for physicians to be aware of this potential ADR, and to closely monitor patients receiving linezolid for the development of BHT. Furthermore, in cases where this ADR occurs, consideration should be given to discontinuing linezolid if required, as optimal treatment outcome is of prime importance. Further research is needed to understand the incidence and clinical significance of linezolid-associated BHT and develop evidence-based guidelines for managing this ADR.
Conflict of interest: Nil
Funding: Nil
References:
- Mishra GP, Mulani J. Implications of bedaquiline-resistant tuberculosis. Lancet Infect Dis [Internet]. 2022 Feb 1 [cited 2022 Jan 27]; 22(2): 166. Available from: http://www.thelancet.com/article/S147330992200007X/fulltext
- World Health Organization (Organization). Consolidated Guidelines on Tuberculosis Treatment. Module 3: Diagnosis. Rapid diagnostics for tuberculosis detection. 2021 update. [Internet]. WHO. 2021. 1–104 p. Available from: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/
- Siddiqui MA, Adil M, Amin SS, et al. Linezolid-induced black hairy tongue: A case report and review of literature. J Dermatology Dermatologic Surg [Internet]. 2022 [cited 2023 Jan 22];26(2):77. Available from: https://www.jddsjournal.org/article.asp?issn=2352-2410; year=2022; volume=26; issue=2; spage=77; epage=79; aulast=Siddiqui
- Lee J, Chung HS, Roh J, et al. Linezolid-induced black hairy tongue in a patient with multidrug-resistant tuberculosis: A case report. Sci Prog [Internet]. 2021 [cited 2023 Jan 22];104(3). Available from: https://pubmed.ncbi.nlm.nih.gov/34541939/
- Balaji G, Maharani B, Ravichandran V, et al. Linezolid induced black hairy tongue. Indian J Pharmacol [Internet]. 2014 Nov 1 [cited 2023 Jan 22]; 46(6): 653. Available from: /pmc/articles/PMC4264085/
- Hashemian SM, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther [Internet]. 2018 [cited 2023 Jan 22]; 12–1759. Available from: http://dx.doi.org/10.2147/DDDT.S164515
- Mishra G. Nix-TB and ZeNix trials: Paving the way for shorter regimens for drug-resistant tuberculosis. Asian Pac J Trop Med [Internet]. 2021 [cited 2023 Jan 22]; 14(10): 431. Available from: https://www.apjtm.org/article.asp?issn=1995-7645;year=2021;volume=14;issue=10;spage=431;epage=432;aulast=Mishra
