Invasion of Human Nails by Microsporum canis
Daiane Flores Dalla Lana1*, Paula Reginatto2, William Lopes3, Marilene Henning Vainstein3, Alexandre Meneghello Fuentefria1,2
1Programa de Pós-Graduação em Ciências Farmacêuticas, Universidade Federal do Rio Grande do Sul, Brazil
2Programa de Pós-graduação em Microbiologia Agrícola e do Ambiente, Universidade Federal do Rio Grande do Sul, Brazil
3Centro de Biotecnologia, Universidade Federal do Rio Grande do Sul, Brazil
Abstract
We report the invasion of the human nail plate by the dermatophytic species Microsporum canis, which was recently described as a biofilm former, in Scanning Electron Microscopy (SEM) images. With the images it was possible to evidence the propagation and aggregation of hyphae on the nails and also the formation of biofilm, as a pathogenicity and virulence factor of the species in cases of onychomycosis.
Dermatophytes are the main etiological agents of onychomycosis, known clinically for the difficult treatment. It is a relevant fungal disease because it may cause paresthesias, itching, pain, difficulties performing activities of daily life, and impair social interactions1. Onychomycosis have a high prevalence worldwide, being very common in older people2,3. Biofilms have currently been associated to the pathogenesis of onychomycosis1. While dermatophytes were previously believed to be planktonic filamentous fungi, recent studies revealed the ability of this group of fungi to form biofilm [4]. Biofilms are admittedly sessile cells aggregates that attach to biological system, such as the nail plate, via an extracellular matrix that encases them1,4. In doing so, they exhibit increased virulence, may develop resistance to antifungal agents, and evade the immune system. Some dermatophytes, mainly referring to the genus Trichophyton form biofilms in vitro5. In addition, Trichophyton rubrum and Trichophyton mentagrophytes begin forming biofilms at 3 hours that are fully formed by 72 hours5. Thus, the biofilm process for Trichophyton spp. is already well defined in the literature5,6. Our research group first reported biofilm formation by Microsporum canis in 20177. Considering this finding, the purpose of this study was to evaluate for the first time the invasion of the human nail plate by this dermatophytic species through scanning electron microscopy (SEM) images.
Healthy nail fragments donated nails of healthy individuals, without fungal disease, were cut into fragments measuring approximately 0.5 cm and washed with distilled water to remove any dirt and debris. Prior to storage, they were autoclaved at 121 °C for 30 minutes to remove any surface contaminations that could influence the tests. They were then kept in tubes sealed at room temperature (28 °C) until use. These fragments of nails were placed on Potato Dextrose Agar (Gibco®) plate. The inoculum of M. canis (MCA 01 clinical isolate, from the Mycology Collection of the Research Group on Applied Mycology, Universidade Federal do Rio Grande do Sul) was added (100 µL, 105 UFC/mL) on the nail fragments on the Potato Dextrose Agar plates. Nails without inoculum were also incubated under the same conditions as control. Subsequently, incubation (35 °C) was performed for 7 days. After this period the samples were prepared for scanning electron microscope (SEM) analysis. The clinical strain of M. canis was identified by the culture macromorphology and the very characteristic micromorphology (direct examination) - spindle-shaped macroconidia that identify the species, together with the patient's symptoms and epidemiological data.
The fixation of the samples was performed with 500 μL of glutaraldehyde (2.5%, type 1, Sigma-Aldrich), diluted with sodium cacodylate (0.1 mol/L, pH 7.2, Sigma-Aldrich), and kept for 1 hour at room temperature8. Then, the samples were washed, postfixed, dehydrated, displaced, dried, sprayed, and chemically dried. Carl Zeiss EVO® SEM (MA10, Oberkochen, Germany), operating at 10kV, was used to observe and photograph the samples, in different magnifications.
SEM images demonstrate the human nail plate without infection (control), in its normal aspect (Fig 1 - A, B, and C) and the nail invasion of the hyphae of M. canis, it being possible to observe ultra-structurally how the pathogens colonize and explore the nail surface (Fig 1 - D, E, and F). The strategy of biofilm formation by M. canis in the human nail is also evident (extracellular matrix; Fig. 1 – E, and F - white arrows indicating), which favors virulence, pathogenicity and resistance1.
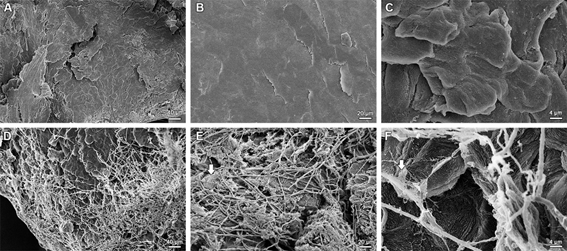
Figure 1: Scanning electron micrographs of human nails invasion by Microsporum canis illustrating biofilm characteristics and details of the nail attack. (A, B, and C) General view of normal human nail, without fungal invasion, in different magnifications. (D, E, and F) Invasion of the hyphae of M. canis on the nail plate, with the observation of the presence of biofilm produced by the species (indicated by white arrows), in different magnifications.
About the mechanisms of nail invasion, Monod et al.9 reported that the pathogenic fungus enter the nail through the lateral nail groove, the distal subungual area, or the dorsal surface of the nail plate causing superficial onychomycosis, or more deeply with under-surface penetration of the nail9. Dermatophyte infections are usually the result of an intertrigo, while repetitive strain injuries to the nail, especially hallux, may play a major role in mould invasion9. In figure 1 it is possible to observe that the ungueal invasion by M. canis occurs throughout the nail plate extension (Fig 1 - D, E, and F), with the fixation of the hyphae superficially and development of the exopolimeric matrix. Research on mechanisms of nail invasion, as well as that of other keratinized tissues, firstly focuses on secreted proteases10,11. All dermatophytes grow well in a medium containing hard keratin as the sole source of carbon and nitrogen, and most secreted proteins in culture supernatant are proteases10,11.
Onychomycosis are recognized an important public health problem, due to its high prevalence and therapeutic problems with elevated rates of progression to chronic lesions and recurrence1. Noteworthy, the cases of nail mycosis caused by M. canis is rare and some are caused by scratching the head (chronic). Therefore, these results corroborate for the better knowledge of the invasion of human nails by dermatophytes, especially for the recently discovered biofilm forming species – M. canis, and consolidates the use of the SEM technique to analyze infectious fungal processes.
References
- Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol. 2019; 80: 835–51.
- Papini M, Piraccini BM, Difonzo E, et al. Epidemiology of onychomycosis in Italy: prevalence data and risk factor identification. Mycoses. 2015; 58: 659–64.
- Vasconcellos C, Pereira CQ, Souza MC, et al. Identification of fungi species in the onychomycosis of institutionalized elderly. An Bras Dermatol. 2013; 88: 377–80.
- Brilhante RSN, Correia EEM, Guedes GMM, et al. Quantitative and structural analyses of the in vitro and ex vivo biofilm-forming ability of dermatophytes. J Med Microbiol. 2017; 66: 1045–52.
- Costa-Orlandi CB, Sardi JC, Santos CT, et al. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling. 2014; 30: 719–27.
- Gupta AK, Daigle D, Carviel JL. The role of biofilms in onychomycosis. J Am Acad Dermatol. 2016; 74: 1241–6.
- Danielli LJ, Lopes W, Vainstein MH, et al. Biofilm formation by Microsporum canis. Clin Microbiol Infect. 2017; 23: 941–2.
- Dalla Lana DF, Carvalho ÂR, Lopes W, et al. Structure-based design of δ-lactones for new antifungal drug development: susceptibility, mechanism of action, and toxicity. Folia Microbiol. 2019. doi: 10.1007/s12223-018-00675-y.
- Monod M, Méhul B. Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. J Fungi. 2019; 20: 1–10.
- Giddey K, Monod M, Barblan J, et al. Comprehensive analysis of proteins secreted by Trichophyton rubrum and Trichophyton violaceum under in vitro conditions. J Proteome Res. 2007; 6: 3081–92.
- Sriranganadane D, Waridel P, Salamin K, et al. Identification of novel secreted proteases during extracellular proteolysis by dermatophytes at acidic pH. Proteomics. 2011; 11: 4422–33.
